Family Support and Training Centers for Lynch Syndrome
Abstruse
Purpose
There are no internationally agreed upon clinical guidelines equally to which women with gynecological cancer would benefit from Lynch syndrome screening or how best to manage the risk of gynecological cancer in women with Lynch syndrome. The Manchester International Consensus Group was convened in April 2017 to address this unmet need. The aim of the Group was to develop clear and comprehensive clinical guidance regarding the management of the gynecological sequelae of Lynch syndrome based on existing evidence and practiced stance from medical professionals and patients.
Methods
Stakeholders from Europe and N America worked together over a two-day workshop to reach consensus on best practice.
Results
Guidance was developed in iv key areas: (1) whether women with gynecological cancer should be screened for Lynch syndrome and (ii) how this should be washed, (three) whether there was a role for gynecological surveillance in women at run a risk of Lynch syndrome, and (4) what preventive measures should exist recommended for women with Lynch syndrome to reduce their hazard of gynecological cancer.
Conclusion
This document provides comprehensive clinical guidance that tin can be referenced by both patients and clinicians so that women with Lynch syndrome tin can wait and receive advisable standards of care.
INTRODUCTION
Lynch syndrome is an autosomal dominantly inherited cancer syndrome including colorectal (CRC), endometrial (EC), and ovarian cancer (OC).1 It is caused by pathogenic variants of the Dna mismatch repair (MMR) system genes MLH1, MSH2, MSH6, and PMS2, which prevent the correction of acquired errors during DNA synthesis. Gynecological cancers are often the sentinel Lynch syndrome event in women and have an excellent prognosis.2 This provides an opportunity to diagnose women before farther oncological sequelae touch them or their family unit. Early diagnosis allows women to be enrolled in cancer surveillance programs and enables pour testing for at-risk relatives. There is well-documented survival reward for those with Lynch syndrome who are compliant with CRC surveillance.3 Farther, early identification of Lynch syndrome can enable the uptake of cancer prevention strategies, including aspirin and take chances-reducing surgery.4,five A timely diagnosis may also have cancer prognosis and treatment implications. For example, MMR-deficient tumors are susceptible to allowed checkpoint inhibition through PD-i occludent.6 These considerations necessitate guidelines to direct the identification and care of individuals affected by Lynch syndrome. Although CRC clinical guidance is widely bachelor, the same is not true for gynecological cancers (eTable i). The lack of comprehensive guidance has led to a nonuniform arroyo to direction of women with Lynch syndrome globally. The aim of the Manchester International Consensus Meeting was to provide the first gynecological-specific guidance for the diagnosis, prevention, and surveillance of Lynch syndrome–associated gynecological malignancies.
The Manchester International Consensus Meeting 2017 for the direction of gynecological cancers in Lynch syndrome
The coming together was held on 24–25 Apr 2017. Fifty stakeholders attended from across Europe and N America, including patients (n = 2), patient back up grouping representatives (n = 2), gynecological oncology surgeons (n = 12), gynecology nurse specialists (due north = five), clinical geneticists (due north = x), genetic counselors (n = 2), medical oncologists (n = 1), colorectal surgeons (n = ii), gastroenterologists (n = ane), histopathologists (n = ten), genetic pathologists (northward = one), health economists (n = 1), and epidemiologists (due north = i).
Preparation for the meeting included a systematic review of the literature to identify key papers to inform discussion. A systematic review with meta-analysis was performed to provide a robust estimate of the prevalence of Lynch syndrome in women with endometrial cancer, using the methodology described in our published protocol.seven The body of literature identified through this search also enabled informed discussion regarding the comparable utility of MMR immunohistochemistry (IHC), microsatellite instability (MSI), MLH1 methylation testing, and direct germline sequencing for pathological variants of the MMR genes by next-generation sequencing (NGS) for Lynch syndrome testing (eTable ii). An identical search was conducted in parallel, substituting the Medical Discipline Heading (MeSH) term "endometrial" with "ovarian." Further searches to identify bear witness for gamble-reducing interventions and the clinical effectiveness of gynecological surveillance in Lynch syndrome (eTable three) were conducted. Key studies were identified by abstract review and graded according to the category of bear witness they achieved (Table 1). Potential bias was assessed past two reviewers, and discrepancies settled by a third, every bit previously described;vii those studies with a high likelihood of bias were excluded. Papers identified through these searches were grouped according to the 4 clinical questions detailed in this document, and sent to the practiced assigned equally question pb before the meeting.
At the coming together, solar day i consisted of 11 lectures covering the prevalence of Lynch syndrome and its associated cancer risks, the patient's perspective, the process of developing clinical guidance, lessons learned from the colorectal community, current diagnostic technologies, and methods of gynecological surveillance. These lectures provided a critical review of the studies identified through the systematic searches described to a higher place and had the purpose of providing the consensus group with up-to-appointment evidence on which to base its recommendations. Participation was encouraged when assessing the quality of the bachelor evidence during the lectures.
The 2nd day rotated delegates through working groups focused on screening for Lynch syndrome in gynecological cancer, diagnostic methods for such screening, the function of run a risk-reducing surgery and gynecological surveillance. Each group benefited from multidisciplinary health-care professional and lay representation. Topics were debated until an agreed statement could be reached. The precise wording of these statements was decided through careful deliberation until unanimous agreement was confirmed past a prove of hands. One time all delegates had rotated through the 4 focus groups, a concluding forum enabled group chairpersons to feed dorsum where consensus had been reached. A farther show of easily was required for individual statements to achieve this consensus certificate. The document was written and edited by the skilful writing group and circulated through all authors until each recommendation was ratified. The levels of recommendation are shown in Table ii.
Question 1: Should women with gynecological cancer be screened for Lynch syndrome? (Box 1)
One in 279 of the general population carry pathogenic variants in ane of the MMR genes, of which the vast majority are unaware.eight Approximately i in 30 CRCs are Lynch syndrome associated.9 The proportion of ECs that are Lynch syndrome associated is around ane in 30, but estimates are based on small studies hampered by methodological limitations. The largest of these (n > 300) are shown in eTable 2.
Current UK guidance from the National Institute for Health and Care Excellence (NICE) supports the universal screening of CRC for Lynch syndrome.10 Given similar rates of Lynch syndrome in EC and CRC, and the potential to reduce mortality through colorectal surveillance and cascade testing of relatives, the Consensus Group strongly recommends that women with EC should also exist screened for Lynch syndrome.
Restricting screening to those with a higher pretest probability of Lynch syndrome (e.g., younger patients) is likely to reduce the resource burden, although at the price of missing more Lynch syndrome cases. I study establish only 25% of IHC MMR-scarce tumors were in women <50 years.11 Hampel et al. establish xx% of proven Lynch cases presented >60 years.12 While older patients may accept a lower risk of Lynch syndrome, and less potential to benefit from risk-reducing measures, they may as well have younger relatives who could benefit from the identification of family pathogenic variants. Farther, targeted screening has its own challenges, particularly that screening is not conducted despite existence indicated.
Amsterdam-Ii and Bethesda criteria are family unit history–based prediction tools designed to target Lynch syndrome screening in CRC. Extrapolation of these tools to EC has shown specificity of 61% and 49% for Amsterdam-II and Bethesda criteria, respectively.xiii Newer prediction tools, MMRpredictone,26, MMRpro, and PREMMv, have increased sensitivity and specificity.fourteen,15,16 Yet, they rely on accurate family cocky-reported history being recorded by the clinician. A quality-controlled family history is time consuming, outside the scope of many busy clinical settings, and does non encounter the specificity or sensitivity needed for a first-line test to identify MMR pathogenic variant carriers,17 however, in the absence of tumor textile, such prediction models can be useful to guide germline testing.
Restricting Lynch syndrome screening to tumors with certain pathological phenotypes, for example endometrioid or clear jail cell morphology, tumors of the lower uterine segment, and those with heavy infiltrates of tumor-associated T-lymphocytes, has not been tested prospectively as a means to directly Lynch syndrome screening.18 A depression torso mass index (BMI) increases the likelihood of Lynch syndrome being the underlying cause in EC.19 In CRC, restricting screening for Lynch syndrome to sure loftier-risk pathological features is not sufficiently sensitive.twenty
With regard to Lynch syndrome–associated OC, there is minimal evidence to guide clinical intendance. A single-center study found 21% of nonserous epithelial OC to be MMR deficient by IHC.21 Lynch syndrome–associated OC is predominantly endometrioid, presenting at an before age and stage than sporadic OC, with improved survival rates.22 Lynch syndrome is plant in vii% of women with synchronous EC and OC.23 Many professional organizations now recommend testing all epithelial OC patients for BRCA1/2 pathological variants.24,25 Given the similar cumulative gamble of OC in Lynch syndrome, testing premenopausal women with epithelial OC for both BRCA1/ii and Lynch syndrome is appropriate. This is even more persuasive in an era of panel gene testing where there is no additional cost to add more genes.
At that place is no bear witness to support a link between Lynch syndrome and other gynecological cancers, neither myometrial, nor squamous cancers of the vulva, vagina, or cervix, in which the most important etiological driver is persistent infection with high-risk human papillomavirus (HPV). Thus screening for Lynch syndrome in women affected by cancers of the lower genital tract is not recommended, with the exception of (HPV-contained) endocervical adenocarcinomas, given the difficulty of distinguishing them from lower uterine segment endometrial cancers.26
Question two: How should women with gynecological cancer be screened for Lynch syndrome? (Box ii)
Screening women with gynecological cancer for Lynch syndrome is a multidisciplinary responsibility. Wellness-care systems require robust procedures for quality-bodacious tumor testing and communication of results. Tissue analysis to triage women for Lynch syndrome testing highlights the potential of having Lynch syndrome rather than diagnosing it. Furthermore, identifying MMR-defective status provides important prognostic information and can straight, for case, immunotherapy treatment strategies.27 Thus, MMR IHC/MSI testing should form part of standard patient intendance and prior consent is not required. When germline testing is recommended, informed consent should be sought and patients are entitled to receive specialist counseling.
Four options were explored for the initial screening of tumor samples for Lynch syndrome: MSI, IHC with methylation testing, MSI with IHC and/or methylation testing, and germline NGS (Fig. 1). NGS is the golden standard for identifying somatic pathogenic variants in MMR genes; there remain challenges in working with formalin-fixed methane series-embedded tumor samples, simply performance of NGS on such samples is improving.28 It should exist noted that exon panel NGS will non identify MLH1 silencing due to methylation.29 Sequencing of PMS2 is problematic due to the presence of numerous pseudogenes.30 NGS is expensive and many hospitals have limited access to it. Thus most studies and current clinical exercise apply a screening triage with the use of IHC and/or MSI before germline NGS and big rearrangement testing (eTable two).
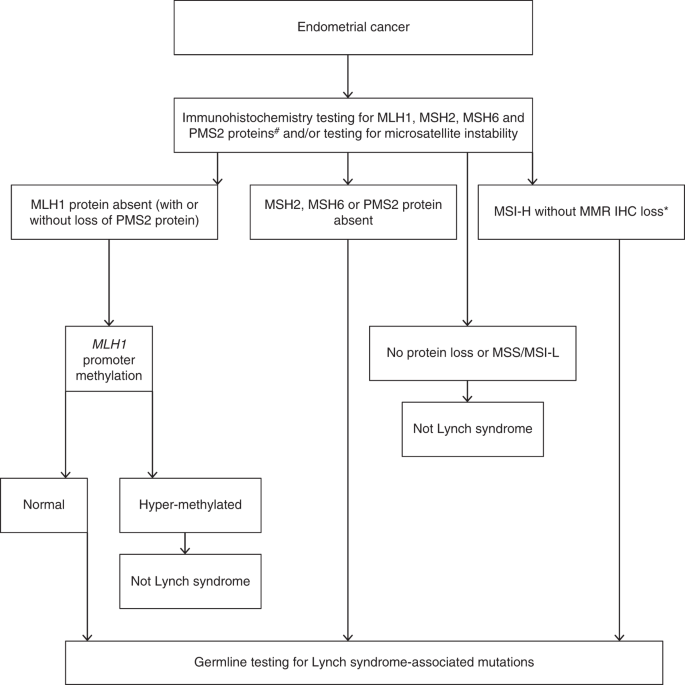
Proposed diagnostic schema of endometrial cancer screening for Lynch syndrome. Consent should exist sought from the patient before germline testing. #We recognize the possibility of using a two poly peptide screen using PMS2 and MSH6 initially, however the evidence base is not definitive. *If only microsatellite testing used without immunohistochemistry then all those found to be MSI-H should either be further triaged with methylation testing or undergo direct germline analysis. IHC immunohistochemistry, MSI-H microsatellite instability loftier, MSI-L microsatellite instability low, MMR mismatch repair, MSS microsatellite stable.
In that location is good cyclopedia between MSI and IHC analysis.31,32 Where there is discordance this may, for example, reflect MSI secondary to POLE pathogenic variants, heterogeneity of MMR loss within the tumor, or microsatellite stable (MSS) MSH6 or PMS2 loss.32,33 The beginning is important considering POLE pathogenic variants accept prognostic implications; the last because MSI triage may miss Lynch syndrome cases due to MSH6 pathogenic variants.34 For IHC, sensitivity and specificity range between 86–100% and 48–67%, respectively.19,35,36 For MSI, sensitivity and specificity are similar at 77–100% and 38–81% (refs. 19,35,36). Where there is a strong family history and where EC/OC presents under the age of fifty years with normal IHC and/or MSI, at that place is still an statement for definitive NGS.37 Investigations should be performed in an agreed stepwise and protocol-driven fashion.ten
IHC analysis has the reward of identifying the specific protein that has been lost, thus indicating the potentially mutated gene. Furthermore, some pathogenic variants in MSH6 have been shown to associate with tumor MSS.38 The identification of MLH1 protein loss enables methylation analysis, which tin exclude women with somatic MLH1 loss from unnecessary NGS.38 Methylation-specific polymerase concatenation reaction (PCR) is simple and cost-effective, still validation work has been near exclusively in the CRC population.39,40 Methylation-specific PCR is not widely available, with only specialist laboratories offering this test. The molecular mechanism for the strong association of BRAF variant with CRC harboring somatic MLH1 hypermethylation is incompletely understood simply appears to be tissue/tumor-specific; unlike algorithms in use for CRC, BRAF immunohistochemistry or sequencing cannot be used every bit a proxy for somatic MLH1 hypermethylation in gynecological cancers, equally oncogenic BRAF variants occur and so rarely in these.41 Therefore, moving direct to germline NGS on the basis of IHC MLH1 loss is also an option, although an expensive 1.
The MMR proteins form heterodimers, with MLH1 pairing with PMS2 and MSH2 pairing with MSH6. These proteins are unstable in their unpaired state, and while MLH1 and MSH2 tin class stable heterodimers with other proteins, PMS2 and MSH6 tin can but dimerize with MLH1 and MSH2 respectively. Information technology has therefore been proposed that IHC analysis tin be performed past testing but ii of the 4 MMR proteins, PMS2 and MSH6, because loss of MLH1 and MSH2 leads to loss of their heterodimer partner (PMS2 and MSH6 respectively).42 However, the accuracy of this arrangement is unproven in gynecological cancers.43 The estimation of stained slides requires an experienced senior clinician, and IHC more frequently needs to be repeated due to uninterpretable staining patterns in EC compared with CRC.44 Furthermore, the upshot of neoadjuvant treatment on tissue analysis for MMR dysfunction has not been defined in EC.45 A reporting proforma is shown in eFigure one. The standing use of tightly regulated and high quality IHC protocols should be assured by laboratory participation in a national or international external quality assurance scheme that covers both IHC methods and interpretation.
A testing strategy using IHC and MLH1 methylation is likely to exist more cost-effective than strategies using MSI testing (because MLH1 methylation must be conducted on all MSI tumors) and strategies non using MLH1 methylation (because methylation testing is cheaper than NGS and excludes a meaning proportion of sporadic cases). Documented IHC results tin besides assist translate NGS results.
Question 3: Is there a role for gynecological surveillance in women at risk of Lynch syndrome? (Box 3)
Many female MMR pathogenic variant carriers opt to undergo gynecological surveillance in lieu of, or whilst awaiting, risk-reducing surgery. The aim of surveillance is to detect premalignant illness or early on phase cancer, with the ultimate aim of improving morbidity and mortality from the malignant gynecological sequelae of Lynch syndrome.
The data relating to gynecological surveillance in Lynch syndrome are generally of low quality, with single-center, retrospective studies predominating (eTable 3). Some studies show benefit and others show no benefit of gynecological surveillance in the early detection of endometrial cancer.46 Survival data are limited and mortality data are defective. However, both preinvasive (atypical hyperplasia) and stage one affliction accept been diagnosed in asymptomatic women undergoing EC surveillance (eTable 3). 1 written report institute that women who were not under surveillance were more likely to die from their EC than those in surveillance, although this did not achieve statistical significance. In addition, three cancers were "missed" in the surveillance group.47 Some other found a high proportion of interval ECs in women undergoing surveillance (6/13) (ref. 48). It is of import to note that EC survival rates in women with Lynch syndrome are extremely good, with 10-year survival of 98% in those undergoing surveillance;3 comparative data for LS-EC in women not undergoing surveillance are not available.
The Consensus Group best-selling that ultrasonography, biopsy, and hysteroscopy could detect EC and premalignant pathological abnormalities. Even so, there is no testify that this leads to a stage shift or improved survival in women with Lynch syndrome–associated EC. Furthermore, many patients identified during gynecological surveillance are symptomatic of endometrial pathology (eTable three). Patient representatives in the Group were potent advocates of gynecological surveillance every bit a ways of regular review and reassurance. Therefore the Consensus Group supported a discussion with individual women as to whether they would wish an annual appointment to undergo detailed symptom inquiry, a rediscussion regarding the option for risk-reducing hysterectomy and bilateral salpingo-oophorectomy and the timing of this, as well as a holistic review of a woman'due south contraceptive and fertility needs, and communication regarding cancer run a risk-reducing behaviors. Women with crimson flag symptoms of gynecological cancer, including aberrant bleeding, weight loss, bloating, change in bowel addiction, recurrent urinary symptoms, and abdominal pain should undergo targeted investigations for gynecological pathology.49,50 The Consensus Group was strongly supportive of the need for rapid admission facilities being bachelor for women with Lynch syndrome and that suspicious symptoms or signs of malignant gynecological disease should not await routine review in dispensary.
Regarding OC, at that place is currently insufficient prove that surveillance is of benefit.46 Large randomized controlled trials (RCTs) of general population screening for OC through ultrasound scanning (USS) or multimodal screening (CA125 analyzed by an algorithm as a first-line examination, followed by reflex USS) have so far failed to demonstrate a statistically significant mortality benefit.51,52 However, screening in high-risk BRCA1/two pathogenic variant carriers with 4-monthly CA125 analyzed by an algorithm and reflex USS does lead to a stage shift in the disease detected; whether this translates into mortality benefit has yet to be established.53 Extrapolation of OC screening enquiry to the LS population is limited by the known biological differences between LS-associated and sporadic/BRCA1/ii-associated OC.54
Whilst there is true equipoise in the literature, high quality research regarding the value of gynecological surveillance in Lynch syndrome could be performed. The options for written report design could include a cluster RCT or a centralized repository for routine data collection from local surveillance programs. Studies should be adequately powered to determine whether surveillance picks up before illness with benefits for patient outcomes, also as assessing its psychological impact and toll-effectiveness.
Question iv: What preventive measures should be recommended in Lynch syndrome to reduce gynecological cancer adventure? (Box four)
Chance-reducing total hysterectomy and bilateral salpingo-oophorectomy prevents gynecological cancer in women at risk of Lynch syndrome.5 Surgery is not without risk and potential long-term side effects, notwithstanding, and preoperative counseling is of import. The laparoscopic arroyo is associated with less postoperative pain, quicker recovery, and improved brusque-term quality of life, making it the preferred approach in simple cases, where resources permit.55 Surgical menopause follows risk-reducing oophorectomy in premenopausal women. This is associated with vasomotor symptoms, urogenital dryness and atrophy, reduced sexual office, emotional lability, and cerebral reject, as well every bit increased risks of osteoporosis, cardiovascular disease and CRC.56 Thus prescription of estrogen-only hormone replacement therapy (HRT) until at least natural menopause age (~ 51 years) is strongly recommended to preclude these sequelae. At that place is some bear witness that prior hysterectomy may be associated with greater discomfort during intubation at colonoscopy, and lower cecal intubation rates.57 Thus it has been suggested that women undergoing colonoscopic surveillance post-obit hysterectomy undergo specific preprocedure counseling and measures to reduce procedural discomfort.58
In that location are skilful quality prospective data outlining the cancer risk associated with specific pathogenic variants and the historic period at which these occur.59 A woman'southward personal risk should be used to provide individualized counseling regarding the need for risk-reducing surgery and the optimal timing of this. According to the Prospective Lynch Syndrome Database (PLSD; http://www.lscarisk.org), the lifetime risk of EC in women with MSH2, MLH1, and MSH6 pathogenic variants is 57%, 43%, and 46%, respectively. The cumulative risk of EC at 40 years of age is 2%, 3%, and 0%, respectively. Lifetime risk for OC in women with MSH2, MLH1, and MSH6 pathogenic variants is 17%, x%, and 13%, respectively. The cumulative chance of OC at xl years of age is 4%, 3%, and four%, respectively.59 Thus MSH6 pathogenic variant carriers may consider undergoing risk-reducing surgery after the age of 40 years, while women with pathogenic variants in either MSH2 or MLH1 may consider risk-reducing surgery at effectually 35 years of historic period bold their childbearing is complete.60 Take chances-reducing surgery at 40 years of age is a toll-effective strategy.61 The take a chance of gynecological cancer in PMS2 carriers is low; however, patient representatives with PMS2 pathogenic variants felt strongly that they should be offered risk-reducing surgery alongside other women with Lynch syndrome.59
In that location is express evidence as to how reproductive and lifestyle factors impact on gynecological cancer gamble in Lynch syndrome. One study suggested that hormonal influences practice modulate cancer risk, however. In addition to combined oral contraceptives, progestin-merely methods (pills, injectable, implants, or intrauterine organization [IUS]) may protect, simply at that place is little supporting bear witness.62 The POET trial looked to explore the use of the IUS for the prevention of EC in women with Lynch syndrome. This trial closed due to poor recruitment without results. Some other study showed an antiproliferative upshot of exogenous progesterone on the endometrium of women with Lynch syndrome, suggesting that these agents could be useful for the chemoprevention of EC.63 Exogenous hormones may also protect against CRC.64
Aspirin reduces incidence of Lynch syndrome–associated EC and other cancers.65 The main toxic effects of aspirin are gastrointestinal. Major gastrointestinal bleeds (those requiring transfusion) are increased in aspirin-takers with an odds ratio (OR) of 1.v–2 (ref. 66). Even so, the absolute rates are small and mainly touch on older individuals. Considering gastrointestinal toxicity is dose dependent, the optimal dose for cancer risk reduction is being explored through the CaPP3 study of 100 mg, 300 mg, or 600 mg/24-hour interval (http://www.capp3.org/).
Smoking, booze employ, and increased BMI may increase the risk of CRC in individuals with Lynch syndrome; however, the impact of lifestyle factors on gynecological cancer adventure is unknown. Aspirin may "normalize" EC chance in obese women with Lynch syndrome.67 Despite lack of robust evidence, women are brash to swallow a healthy diet, avoid obesity, take regular exercise, avert smoking, only drink alcohol in moderation, and avoid known carcinogens as office of maintaining healthy lifestyles.
Discussion
This is the first gynecology-focused internationally agreed-upon clinical guideline for the care of women with or at risk of Lynch syndrome.
Our key recommendations are as follows: (1) all stakeholders should be informed of the affect of Lynch syndrome on gynecological cancer take a chance; (two) systems should exist established to screen for Lynch syndrome in women with endometrial cancer; (iii) women at risk of Lynch syndrome should exist offered take a chance-reducing hysterectomy with bilateral salpingo-oophorectomy, at a time appropriate to them; and (4) further inquiry is required to establish the value of gynecological cancer surveillance in Lynch syndrome and to explore other key areas where there is currently scarce evidence to define advisable standards of care. Screening for Lynch syndrome is only recommended if effective management exists to benefit those who screen positive.
The strength of our guidance comes from the wide and expert medical specialty representation that forms our Consensus Group. All relevant stakeholders, including patients and patient advocates, were given equal voice during discussion. Despite different perspectives and expertise, we were able to achieve a consensus view on topics of international importance. Past focusing solely on the gynecological aspects of Lynch syndrome, we were able to provide the most comprehensive guidelines yet for the empowerment of both clinicians and patients. This was all achieved without corporate sponsorship.
The major limitation of our work is the lack of robust bear witness on which to base our discussions and recommendations. It is hoped that these guidelines will provide the much-needed impetus to inspire researchers and funders to undertake and commission loftier quality enquiry to fill these gaps in our understanding.
References
-
Lynch HT, Lynch PM, Lanspa SJ, Snyder CL, Lynch JF, Boland CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76:1–18.
-
Lu KH, Dinh M, Kohlmann W, et al. Gynecologic cancer as a "scout cancer" for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol. 2005;105:569–574.
-
Møller P, Seppälä T, Bernstein I, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: get-go report from the prospective Lynch syndrome database. Gut. 2017;66:464–472.
-
Burn J, Gerdes A-One thousand, Macrae F, et al. Long-term event of aspirin on cancer risk in carriers of hereditary colorectal cancer: an assay from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081–2087.
-
Schmeler KM, Lynch HT, Chen 50-K, et al. Safety surgery to reduce the chance of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–269.
-
Le DT, Uram JN, Wang H, et al. PD-1 occludent in tumors with mismatch-repair deficiency. Northward Engl J Med. 2015;372:2509–2520.
-
Ryan NAJ, Blake D, Cabrera-Nifty G, Glaire MA, Evans DG, Crosbie EJ. The prevalence of Lynch syndrome in women with endometrial cancer: a systematic review protocol. Syst Rev. 2018;vii:121
-
Hampel H, la Chapelle de A. The search for unaffected individuals with Lynch syndrome: do the ends justify the means? Cancer Prev Res (Phila). 2011;4:one–5.
-
Moreira Fifty, Balaguer F, Lindor Due north, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555–1565.
-
The National Institute for Health and Care Excellence. Molecular testing strategies for Lynch syndrome in people with colorectal cancer. Diagn Guidel. 2017;27:1–37. https://www.nice.org.united kingdom/guidance/dg27
-
Mills AM, Liou S, Ford JM, Berek JS, Pai RK, Longacre TA. Lynch syndrome screening should be considered for all patients with newly diagnosed endometrial cancer. Am J Surg Pathol. 2014;38:1501–1509.
-
Hampel H, Frankel W, Panescu J, et al. Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) amid endometrial cancer patients. Cancer Res. 2006;66:7810–7817.
-
Syngal S, Fox EA, Eng C, Kolodner RD, Garber JE. Sensitivity and specificity of clinical criteria for hereditary not-polyposis colorectal cancer associated mutations in MSH2 and MLH1. J Med Genet. 2000;37:641–645.
-
Barnetson RA, Tenesa A, Farrington SM, et al. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. Northward Engl J Med. 2006;354:2751–2763.
-
Chen Due south, Wang W, Lee S, et al. Prediction of germline mutations and cancer hazard in the Lynch syndrome. JAMA. 2006;296:1479–1487.
-
Kastrinos F, Uno H, Ukaegbu C, et al. Development and Validation of the PREMM5 Model for Comprehensive Run a risk Cess of Lynch Syndrome. J Clin Oncol. 2017:JCO2016696120.
-
Sjursen W, Haukanes BI, Grindedal EM, et al. Current clinical criteria for Lynch syndrome are not sensitive plenty to place MSH6 mutation carriers. J Med Genet. 2010;47:579–585.
-
Lu HK, Broaddus RR. Gynecologic cancers in Lynch syndrome/HNPCC. Fam Cancer. 2005;4:249–254.
-
Lu KH, Schorge JO, Rodabaugh KJ, et al. Prospective determination of prevalence of Lynch syndrome in immature women with endometrial cancer. J Clin Oncol. 2007;25:5158–5164.
-
Greenson JK, Bonner JD, Ben-Yzhak O, et al. Phenotype of microsatellite unstable colorectal carcinomas: well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol. 2003;27:563–570.
-
Chui MH, Ryan P, Radigan J, et al. The histomorphology of Lynch syndrome-associated ovarian carcinomas toward a subtype-specific screening strategy. Am J Surg Pathol. 2014;38:1173–1181.
-
Ryan NAJ, Evans DG, Green Thou, Crosbie EJ. Pathological features and clinical behavior of Lynch syndrome-associated ovarian cancer. Gynecol Oncol. 2017;0:491–495.
-
Soliman PT, Slomovitz BM, Broaddus RR, et al. Synchronous primary cancers of the endometrium and ovary: a unmarried establishment review of 84 cases. Gynecol Oncol. 2004;94:456–462.
-
Capoluongo E, Ellison Yard, López-Guerrero JA, et al. Guidance statement on BRCA1/2 tumor testing in ovarian cancer patients. Semin Oncol. 2017;44:187–197.
-
Vergote I, Banerjee S, Gerdes A-Thousand, et al. Electric current perspectives on recommendations for BRCA genetic testing in ovarian cancer patients. Eur J Cancer. 2016;69:127–134.
-
Westin SN, Lacour RA, Urbauer DL, et al. Carcinoma of the lower uterine segment: a newly described association with Lynch syndrome. J Clin Oncol. 2008;26:5965–5971.
-
Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-i blockade. Science. 2017;357:409–413.
-
Richman SD, Fairley J, Butler R, Deans ZC. RAS screening in colorectal cancer: a comprehensive analysis of the results from the UK NEQAS colorectal cancer external quality balls schemes (2009–16). Virchows Curvation. 2017;471:721–729.
-
Foley AR, Donkervoort S, Bönnemann CG. Next-generation sequencing still needs our generation'south clinicians. Neurol Genet. 2015;1:e13.
-
Vaughn CP, Hart KJ, Samowitz WS, Swensen JJ. Avoidance of pseudogene interference in the detection of 3′ deletions in PMS2. Hum Mutat. 2011;32:1063–1071.
-
Stewart A PhD. Genetic Testing Strategies in Newly Diagnosed Endometrial Cancer Patients Aimed at Reducing Morbidity or Mortality from Lynch Syndrome in the Index Case or Her Relatives. PLoS Curr. 2013. Accessed 25 March 2019 https://doi.org/x.1371/currents.eogt.b59a6e84f27c536e50db4e46aa26309c.
-
Stelloo E, Jansen AML, Osse EM, et al. Applied guidance for mismatch repair-deficiency testing in endometrial cancer. Ann Oncol. 2017;28:96–102.
-
Miyaki Thou, Konishi One thousand, Tanaka K, et al. Germline mutation of MSH6 as the cause of hereditary nonpolyposis colorectal cancer. Nat Genet. 1997;17:271–272.
-
Cancer Genome Atlas Inquiry Network, et al. Integrated genomic label of endometrial carcinoma. Nature. 2013;497:67–73.
-
Mercado RC, Hampel H, Kastrinos F, et al. Performance of PREMM(1,2,6), MMRpredict, and MMRpro in detecting Lynch syndrome among endometrial cancer cases. Genet Med. 2012;14:670–680.
-
Berends MJW, Wu Y, Sijmons RH, et al. Toward new strategies to select young endometrial cancer patients for mismatch repair gene mutation analysis. J Clin Oncol. 2003;21:4364–4370.
-
Resnick KE, Hampel H, Fishel R, Cohn DE. Current and emerging trends in Lynch syndrome identification in women with endometrial cancer. Gynecol Oncol. 2009;114:128–134.
-
Goodfellow PJ, Billingsley CC, Lankes HA, et al. Combined microsatellite instability, MLH1 methylation assay, and immunohistochemistry for Lynch syndrome screening in endometrial cancers from GOG210: an NRG Oncology and Gynecologic Oncology Group report. J Clin Oncol. 2015;33:4301–4308.
-
Herman JG, Graff JR, Myöhänen Due south, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR analysis for methylation status of CpG islands. Proc Natl Acad Sci United states. 1996;93:9821–9826.
-
Snowsill T, Coelho H, Huxley N, et al. Molecular testing for Lynch syndrome in people with colorectal cancer: systematic reviews and economic evaluation. Wellness Technol Appraise. 2017;21:1–238.
-
Metcalf AM, Spurdle AB. Endometrial tumour BRAF mutations and MLH1 promoter methylation as predictors of germline mismatch repair gene mutation status: a literature review. Fam Cancer. 2013;13:1–12.
-
Mojtahed A, Schrijver I, Ford JM, Longacre TA, Pai RK. A two-antibiotic mismatch repair poly peptide immunohistochemistry screening approach for colorectal carcinomas, peel sebaceous tumors, and gynecologic tract carcinomas. Modern Pathol. 2011;24:1004–1014.
-
Torlakovic EE, Cheung CC, D'Arrigo C, et al. Development of quality assurance for clinical immunohistochemistry in the era of precision medicine. Part iii: technical validation of immunohistochemistry (IHC) assays in clinical IHC laboratories. Appl Immunohistochem Mol Morphol. 2017;25:151–159.
-
Modica I, Soslow RA, Black D, Tornos C, Kauff Due north, Shia J. Utility of immunohistochemistry in predicting microsatellite instability in endometrial carcinoma. Am J Surg Pathol. 2007;31:744–751.
-
Kamat N, Khidhir MA, Hussain S, Alashari MM, Rannug U. Chemotherapy induced microsatellite instability and loss of heterozygosity in chromosomes 2, five, ten, and 17 in solid tumor patients. Cancer Cell Int. 2014;14:118
-
Auranen A, Joutsiniemi T. A systematic review of gynecological cancer surveillance in women belonging to hereditary nonpolyposis colorectal cancer (Lynch syndrome) families. Acta Obstet Gynecol Scand. 2011;90:437–444.
-
Renkonen-Sinisalo Fifty, Bützow R, Leminen A, Lehtovirta P, Mecklin J-P, Järvinen HJ. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int J Cancer. 2007;120:821–824.
-
Ketabi Z, Gerdes A-M, Mosgaard B, Ladelund S, Bernstein I. The results of gynecologic surveillance in families with hereditary nonpolyposis colorectal cancer. Gynecol Oncol. 2014;133:526–530.
-
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Daraï Due east. Endometrial cancer. Lancet. 2016;387:1094–1108.
-
Funston Yard, O'Flynn H, Ryan NAJ, Hamilton West, Crosbie EJ. Recognizing gynecological cancer in principal care: take a chance factors, red flags, and referrals. Adv Ther. 2018;35:577–589.
-
Jacobs IJ, Menon U, Ryan A, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387:945–956.
-
Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305:2295–2303.
-
Rosenthal AN, Fraser LSM, Philpott S, et al. Evidence of stage shift in women diagnosed with ovarian cancer during Stage II of the United kingdom of great britain and northern ireland Familial Ovarian Cancer Screening Study. J Clin Oncol. 2017;35:1411
-
Ryan NAJ, Bolton J, McVey RJ, Evans DG, Crosbie EJ. BRCA and Lynch syndrome-associated ovarian cancers behave differently. Gynecol Oncol Rep. 2017;22:108–109.
-
Garry R, Fountain J, Mason S, et al. The eVALuate written report: ii parallel randomised trials, ane comparing laparoscopic with abdominal hysterectomy, the other comparing laparoscopic with vaginal hysterectomy. BMJ. 2004;328:129.
-
Rymer J, Morris EP. Extracts from 'clinical prove': menopausal symptoms. BMJ. 2000;321:1516–1519.
-
Clancy C, Burke JP, Chang KH, Coffey JC. The effect of hysterectomy on colonoscopy completion: a systematic review and meta-assay. Dis Colon Rectum. 2014;57:1317–1323.
-
Dyson JK, Bricklayer JM, Rutter MD. Prior hysterectomy and discomfort during colonoscopy: a retrospective cohort analysis. Endoscopy. 2014;46:493–498.
-
Møller P, Seppälä TT, Bernstein I, et al. Cancer risk and survival inpath_MMRcarriers past gene and gender up to 75 years of historic period: a report from the Prospective Lynch Syndrome Database. Gut. 2017;67:1306–1316.
-
Ryan NAJ, Morris J, Green K, et al. Clan of Mismatch Repair Mutation With Age at Cancer Onset in Lynch Syndrome: Implications for Stratified Surveillance Strategies. JAMA Oncol. 2017;3:1702–1706.
-
Kwon JS, Lord's day CC, Peterson SK, et al. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome. Cancer. 2008;113:326–335.
-
Dashti SG, Chau R, Ouakrim DA, et al. Female hormonal factors and the risk of endometrial cancer in Lynch syndrome. JAMA. 2015;314:61–71.
-
Lu KH, Loose DS, Yates MS, et al. Prospective multicenter randomized intermediate biomarker study of oral contraceptive versus Depo-Provera for prevention of endometrial cancer in women with Lynch syndrome. Cancer Prev Res. 2013;6:774–781.
-
Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2009;350:991–1004.
-
Rothwell PM, Fowkes FGR, Belch JF, Ogawa H, Warlow CP, Meade TW. Result of daily aspirin on long-term risk of expiry due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41.
-
Roderick PJ, Wilkes HC, Meade TW. The gastrointestinal toxicity of aspirin: an overview of randomised controlled trials. Br J Clin Pharmacol. 1993;35:219–226.
-
Movahedi M, Bishop DT, Macrae F, et al. Obesity, aspirin, and risk of colorectal cancer in carriers of hereditary colorectal cancer: a prospective investigation in the CAPP2 Study. J Clin Oncol. 2015;33:3591–3597.
Acknowledgements
We would similar to acknowledge Laura Gordon, Christine Dark-brown, and Angela Cooke from the University of Manchester, UK for the administrative support they provided for the Consensus coming together. E.J.C. was supported through a National Constitute for Health Enquiry (NIHR) Clinician Scientist Award (NIHR-CS-012–009), N.A.J.R. was supported through a Medical Research Council (MRC) Doctoral Research Fellowship (MR/M018431/ane), and D.Yard.E is an NIHR Senior Investigator (NF-SI- 0513–10076). E.J.C. and D.G.E are supported by the NIHR Manchester Biomedical Research Centre (IS-BRC-1215–20007). Researchers at University Higher London are supported by the NIHR Biomedical Research Heart at Academy College London Hospitals NHS Foundation Trust and Academy College London. This article presents independent enquiry funded by the NIHR and MRC. The views expressed are those of the authors and non necessarily those of the MRC, NHS, NIHR, or the Department of Wellness.
Writer information
Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosure
The authors declare no conflicts of interest.
Additional information
Publisher'south notation: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of the Manchester International Consensus Grouping are listed in the Acknowledgements
Supplementary information
Rights and permissions
Open up Access This commodity is licensed under a Artistic Commons Attribution 4.0 International License, which permits use, sharing, accommodation, distribution and reproduction in any medium or format, as long equally you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third political party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended apply is not permitted past statutory regulation or exceeds the permitted use, y'all volition demand to obtain permission direct from the copyright holder. To view a re-create of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
Virtually this commodity
Cite this article
Crosbie, Due east.J., Ryan, Northward.A.J., Arends, M.J. et al. The Manchester International Consensus Group recommendations for the direction of gynecological cancers in Lynch syndrome. Genet Med 21, 2390–2400 (2019). https://doi.org/x.1038/s41436-019-0489-y
-
Received:
-
Accustomed:
-
Published:
-
Issue Date:
-
DOI : https://doi.org/10.1038/s41436-019-0489-y
Keywords
- Lynch syndrome
- endometrial cancer
- screening
- surveillance
- guidance
Further reading
winkfieldsuchatedly.blogspot.com
Source: https://www.nature.com/articles/s41436-019-0489-y
0 Response to "Family Support and Training Centers for Lynch Syndrome"
Post a Comment